Fluorinated Compounds
Last Friday I was fortunate enough to be present at my girlfriend’s Ph.D. thesis defence at the University of Copenhagen. In case anyone is wondering, it went very well, and all the opponents spoke highly of her work, and now we can all call her “Doctor”. I have incidentally included a small photo gallery from the day of the defence at the bottom of this post. However, what I really want to talk about is the subject matter of the thesis itself.
As you may have guessed from the title, the thesis is concerned with fluorinated compounds. The full name of the thesis is Polyfluorinated surfactants in food packaging of paper and board. The research investigates the implications of fluorinated compounds when they are used in food packaging. After the high-profile banning of BPA from water bottles, it doesn’t take a huge leap of imagination to come up with ways in which other chemicals in food packaging might have adverse effects.
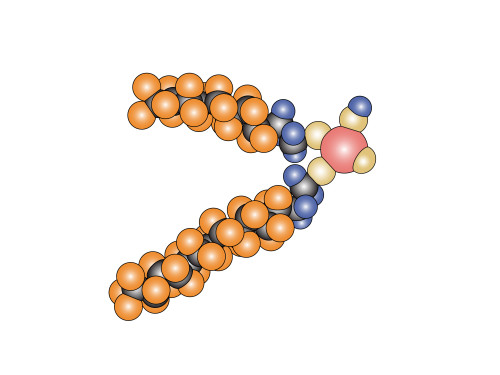
Fortunately (or unfortunately) I have had the privilege of reading through the thesis many times, at various stages of its development. It is written in English, and being a native English speaker, I have some amount of usefulness in spotting odd uses of grammar, and finding better choices of words in certain situations. To be honest, I didn’t have to correct very much. Most Danes speak English quite fluently, and unsurprisingly, Danes who have been in educational institutions for long enough to get a PhD speak and write better English than many of my Australian friends for whom English is their ONLY language. (I also drew the molecules for her thesis, including the cover image above).
Anyway, while many (indeed most) of the more technical points of chemistry went straight over my head, I was able to quite easily follow what was going on in the thesis, and I write about it here not only because I find it interesting, but also because I find it concerning from a public health perspective.
So what are fluorinated compounds? They are chemicals with the chemical element fluorine in them. The ones being investigated are ones that have long chains of CF2, that is a carbon atom with two fluorines attached. The carbon acts as a backbone while the fluorine attaches to the outside (they’re the orange blobs in the drawing above, the carbons are black). They’re very useful because the bonds don’t break down easily, and they have the property of being both hydrophobic and lipophobic, which means that they repel both water-based solutions as well as oil-based solutions. Teflon (polytetrofluoroethylene) is a good example of a commonly used fluorinated compound.
Imagine a popcorn bag. In the past, simple popcorn bags were coated with wax so that the paper in the bag wouldn’t degrade too quickly after coming into contact with the butter in the popcorn. This was all well and good until we invented microwave popcorn. Regular wax breaks down in a microwave, so you have to coat the inside of the bag with something that will withstand the heat, and this is where materials like fluorinated compounds come in.
So what’s the problem? As mentioned above, they are useful because the carbon-fluorine bonds are strong and don’t break down easily. It is also for precisely this reason that these compounds accumulate in nature. It gets worse though, these compounds are bioaccumulative – they accumulate in living organisms. It was at this point where I was surprised to learn that most people living in the western world live with significant amounts of chemicals and plastics in their bodies that have slowly accumulated over time.
Many of these substances are mostly harmless, but the long-term effects of most of them are unknown. There is mounting evidence now that fluorinated compounds fall into the category of being endocrine disruptors, meaning that (like BPA) they can disrupt your hormones. That has a number of bad effects, notable among them being lower sperm counts (leading to infertility) as well as babies being born with underdeveloped genitalia.
So this was the crux of the thesis – do fluorinated compounds used in food packaging contaminate our food? It turns out that finding an answer to this question requires some impressive instrumental trickery and knowledge of chemistry. I used to think that you just put a bunch of samples through a very large and expensive machine and then it would tell you if what you were looking for was there, and how much there was. Turns out that there’s quite a lot more to it.
The short answer is yes. In most cases, there was significant migration (that’s what they call it) of these compounds from the packaging to the food. We should all be concerned. Since this result would be considered quite recent, there has not been sufficient time to develop and implement regulations on fluorinated compounds in food packaging materials. In the meantime, we should make an attempt to avoid exposure to them wherever possible.
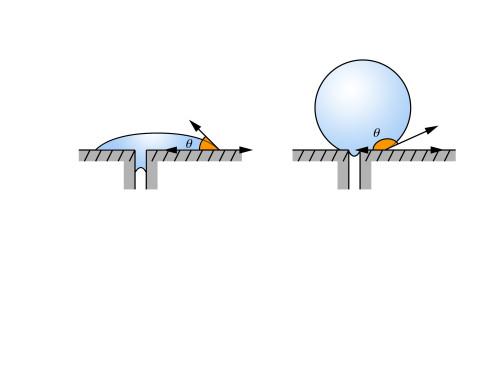
But how? First we have the droplet test. Since fluorinated compounds are very strong surfactants, they have a very high surface energy. This causes droplets on the surface to form into tight balls. In particular, pay close attention to the angle of contact between the droplet and the surface. In the diagram above, the droplet on the right would indicate that fluorinated compounds were being used.
The next test is called the “tear test”. Since these compounds are used to impregnate paper and (card)board, the materials can be torn. When the packaging material is torn, pay close attention to where the tear takes place. If there is a separation, that is – if there is one layer of paper and a separate layer of clear plastic, then there’s no need to worry about fluorinated compounds. In this case, you have a plastic coating, and plastics have been around for longer and have regulations in place. If, however, you find that there is no separation, then you most likely have yourself a fluorinated compound.
Don’t despair though, there are varying degrees of badness. The greatest amount of migration tends to occur when the packaging is used on wet, and especially greasy foods. Also of concern is when the content of the packaging is intended to be heated with the packaging still in contact with it (remember those bags of microwave popcorn). Thirdly, more flexible packaging materials, like thin paper, are worse because they require a higher amount of fluorinated compounds to effectively impregnate them. For reasons that should be obvious, the longer something is in contact with its fluorinated compound-impregnated packaging material, the worse you can expect it to be. Dried foods and frozen foods are often ok though.
So there you have it, advance notice on the next widely-used chemical that will probably eventually get banned. Just the tip of the iceberg in the slow chemical contamination of our biosphere. And now, as promised, photos from the day of the thesis defence (click the images for a larger view):




























Leave a comment